|
Approximations
for Liquids:
|
|
| Specific Volume | |
| Specific Internal Energy | |
| Specific Enthalpy | |
As shown in the figure on the right, when the ratios |
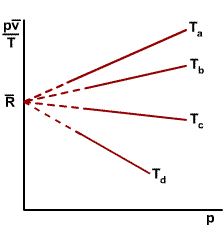 |
||
|
|
Eqn.
(*)
|
||
|
where
|
|||
| M = molecular weight | |||
Note: ![]() is
the universal gas constant, and it is the same for all gases.
is
the universal gas constant, and it is the same for all gases.
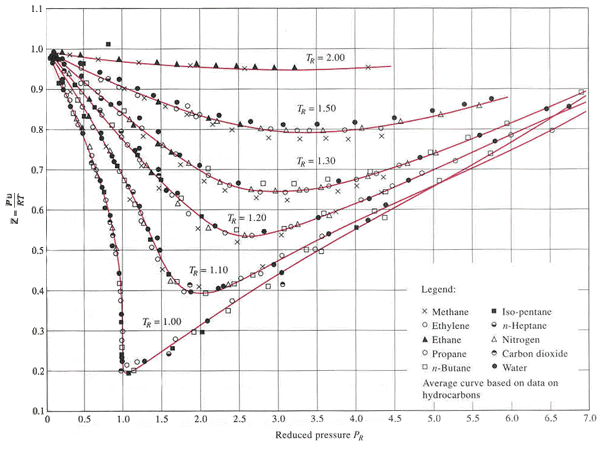
Compressibility
chart for various gases
Reference:
Cengel, Y. A. and Boles, M. A., "Thermodynamics: An Engineering Approach",
3rd ed., WCB/McGraw-Hill, Boston, 1998.
The compressibility factor
is defined as ![]() .
.
Eqn (*) can then be written in terms of the compressibility factor as
follows:
![]()
![]()
From the compressibility chart,
it can be observed that ![]() when the reduced pressure
when the reduced pressure![]() and the reduced temperature
and the reduced temperature ![]() .
.
[![]() and
and ![]() are the
critical pressure and
critical temperature, respectively.]
are the
critical pressure and
critical temperature, respectively.]
![]()
![]()
![]() ... and
B, C, D, ... are virial coefficients which account for the force interactions
among molecules. Z measures
the departure from the ideal gas equation of state.
When
... and
B, C, D, ... are virial coefficients which account for the force interactions
among molecules. Z measures
the departure from the ideal gas equation of state.
When ![]() , the
force interactions between molecules are no longer significant.
, the
force interactions between molecules are no longer significant.
|
Ideal
Gas
|
||
| - The gas consists of molecules that are in random motion and obey the laws of mechanics. | ||
| - The total number of molecules is large, but the volume of the molecules is a negligibly small fraction of the volume occupied by the gas. | ||
| - No appreciable forces act on the molecules except during the collisions. |
|
Equation
of State (Z=1)
|
|
| Equations in Different Forms: | pv = RT |
| pV = mRT | |
| PV = nRT | |
| Note: |
Equation of state requires the use of absolute temperature and absolute pressure. |
| For Ideal Gases: | u = u(T) |
| h = h(T) = u(T) + pv = u(T) + RT | |
|
Specific
Heat
|
Energy required to raise the temperature of a unit mass of a substance by one degree |
|
Specific
Heat at Constant Volume
|
|
|
Specific
Heat at Constant Pressure
|
|
|
Specific
Heat Ratio
|
|
|
For
Ideal Gas:
|
|
|
Specific
Heat at Constant Volume
|
|
|
Specific
Heat at Constant Pressure
|
|
|
Relationship
Between Specific Heat at Constant Volume and Constant Pressure
|
From
the definition of the enthalpy, we have h = u + pv = u + RT |
|
Expressed
in Terms of Specific Heat Ratio
|
Since |
|
Ideal
Gas
|
||
|
Specific
Enthalpy
|
where Therefore, we have |
|
|
Polynomial
Form of Specific Heat at Constant Pressure as a Function of Temperature
|
|
|
|
Change
in Specific Internal Energy
|
|
|
|
Change
in Specific Enthalpy
|
||
|
Specific
Heat at Constant Volume
|
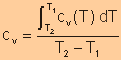 |
OR
use the specific heat evaluated at the average temperature over the interval. |
|
Specific
Heat at Constant Pressure
|
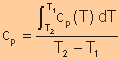 |
|
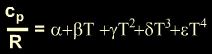 |
|||||
|
where
T is in Kelvin and the above equation is valid where 300
K < T < 1000 K
|
|||||
|
Gas
|
|
|
|
|
|
|
CO
|
3.710
|
-1.619
|
3.692
|
-2.032
|
0.240
|
|
|
2.401
|
8.735
|
-6.607
|
2.002
|
0
|
|
|
3.057
|
2.677
|
-5.810
|
5.521
|
-1.812
|
|
|
4.070
|
-1.108
|
4.152
|
-2.964
|
0.807
|
|
|
3.626
|
-1.878
|
7.055
|
-6.764
|
2.156
|
|
|
3.675
|
-1.208
|
2.324
|
-0.632
|
-0.226
|
|
Air
|
3.653
|
-1.337
|
3.294
|
-1.913
|
0.2763
|
|
|
3.267
|
5.324
|
0.684
|
-5.281
|
2.559
|
|
|
3.826
|
-3.979
|
24.558
|
-22.733
|
6.963
|
|
|
1.410
|
19.057
|
-24.501
|
16.391
|
-4.135
|
|
|
1.426
|
11.383
|
7.989
|
-16.254
|
6.749
|
|
Monatomic
Gases
|
2.5
|
0
|
0
|
0
|
0
|
Reference: Moran, M. J. and Shapiro, H. N., "Fundamental of Engineering Thermodynamics," 2nd ed., John Wiley & Sons, Inc., New York, 1992. pp.718 |
|||||
|
Polytropic
Process
|
|
|
Equation
Form
|
|
|
Work
Done (
|
|
|
Work
Done (n=1)
|
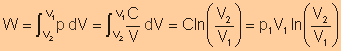 |
|
Polytropic
Process of an Ideal Gas
|
|
|
Work
Done (
|
|
|
Work
Done (n=1)
|
|
|
Relationship
of T with p and V
|
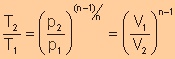 (Derivations are shown in the next table)
(Derivations are shown in the next table) |
|
Derivations
|
|
|
Relationship
Between T and V
|
Relationship
Between T and p
|
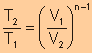 |
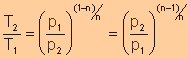 |