The first law of thermodynamics requires that energy be conserved during a process, but place no restriction on the direction of a process. Satisfying the first law does not ensure that a process will actually occur.
The inadequacy of the first law to identify whether a process can take place is remedied by introducing the second law of thermodynamics. With the help of a property, called entropy, we can identify if a process violate the second law.
A process will not occur unless it satisfies both the first and the second laws of thermodynamics.
The first law is concerned with the quantity of energy only.
| The second law asserts that: | ||
| 1. Process occurs in a certain direction. |
 |
|
| 2. Energy has quality as well as quantity. |
 |
|
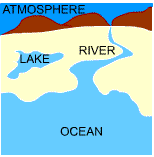
| Thermal energy reservoir is a hypothetical body with a relatively large thermal energy capacity that can supply or absorb finite amount of energy as heat without undergoing any change in temperature. Examples of thermal energy reservoir are atmosphere, lake, river, and ocean. |
 |
| A reservoir that supplies energy in the form of heat is called a source, and one that absorbs energy in the form of heat is called a sink. |
|
|
| Mismanagement of the waste energy can significantly increase the temperature of the environment. It can disrupt marine life in the oceans, lakes and rivers. On the other hand, a careful handling of the waste energy, one can actually improve the quality of marine life by keeping the temperature at a desirable level. |
|
|
| Work can be converted to heat directly and completely, but converting heat to work requires the use of some devices. These devices are called heat engines. |
|
|
|
|
Characteristics
of Heat Engines:
|
||
| 1. They receive heat from a high-temperature source. | ||
| 2. They convert part of this heat to work. | ||
| 3. They reject the remaining waste heat to low-temperature sink. | ||
| 4. They operate on a cycle. |
Thermodynamic cycle
Mechanical cycle - the working fluid does not undergo a complete cycle.
Components of a power plant:
open systems
Power plant: closed system
![]()
Thermal Efficiency
| The extent
of the energy conversion from heat to work |
|||||||||||
|
|||||||||||
|
Kelvin-Planck
Statement
|
|
| It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce an equivalent amount of work. |
A heat engine must exchange heat with a low-temperature sink as well as a high-temperature source to keep operating.
No heat engine can have a thermal efficiency of 100%.
Note that the impossibility
of having a 100 percent efficiency heat engine is not due to friction
or other dissipative effects.
|
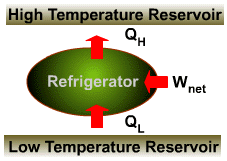
The transfer of heat from a low-temperature medium to a high- temperature one requires special devices called refrigerators (heat pumps). Refrigerators, like heat engines, are cyclic devices. The working fluid in the refrigeration cycle is called a refrigerant. |
|
|
Freezer compartment :
evaporator |
 |
Coefficient of Performance
![]()
| Refrigerators and heat pumps operate on the same cycle but differ in their objectives. | |
|
|
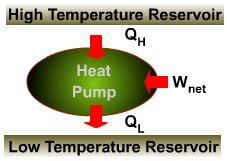 |
Coefficient of Performance
![]()
![]()
Heat Pumps COP: 2-3
Air Conditioners : Refrigerators
|
Clausius
Statement
|
|
| It is impossible to construct a device that operates on a cycle to and produces no effect other than the transfer of heat from a lower temperature body to a higher temperature body. |
A violation of the Kelvin-Planck statement leads to the violation of the
Clausius statement
A violation of the Clausius statement leads to the violation of the Kelvin-Planck statement
 |
 |
 |