Three most important intensive properties in engineering thermodynamics
- Specific Volume
- Pressure
- Temperature
Continuum Hypothesis - A substance is viewed to be a continuous, homogeneous matter. This idealization is valid as long as the length scale one deals with is large relative to the intermolecular spacings.
![]()
V' is the smallest volume for which the matter can be considered a continuum and is normally small enough that it can be consider a point.
V' contains enough particles for statistical averages to be significant.
Specific Volume - reciprocal of the density,
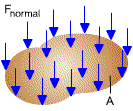
Pressure - the normal force exerted by a fluid per unit area
|  | |
For
a fluid at rest, pressure at a given point is the same in all directions. The
pressure in a fluid increases with depth as a result of the weight of the fluid.
The pressure varies in the vertical direction as a result of gravitational effects,
but there is no variation in the horizontal direction. The pressure in a tank
containing a gas may be considered to be uniform since the weight of the gas is
too small to make a significant difference.
-
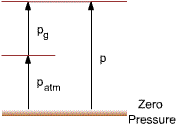
the actual pressure at a given position.
- it is measured relative to absolute
vacuum; i.e., absolute zero pressure.
* Absolute pressure must be
used in thermodynamic relations.
|
Readings from a pressure-measuring
device
|  | |
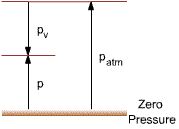
the pressure in the system < the local atmospheric pressure
|  | |
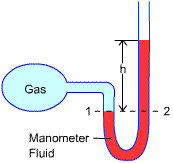
Manometer
- U-tube containing a fluid such as mercury, water, alcohol or oil. Since the
gravitational effects of gases are negligible, the pressure anywhere in the tank
and at position 1 has the same value. Furthermore, since pressure in a fluid does
not vary in the horizontal direction within a fluid, the pressure at 2 is the
same as the pressure at 1.
|  | ||||
-
a device used to measure the atmospheric pressure
| 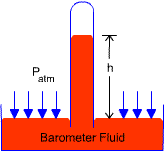 | |
2.6.6 Standard Atmosphere
-
the pressure produced by a column of mercury 760 mm (29.92 in) in height at 0
![]() under
standard gravitational acceleration (g = 9.807 m/s2)
under
standard gravitational acceleration (g = 9.807 m/s2)
![]()
if
a water column is used instead, it would require 10.3 m.