Temperature - a concept originates with our sense perceptions.
Several properties of materials
change with temperature in a repeatable and predictable way, and this
forms the basis for accurate temperature.
Examples: volume expansion (mercury), electrical resistance.
Any body with at least one measurable property that changes with temperature can be used as a thermometer. Such a property is called thermometric property. The particular substance that exhibits changes in the thermometric property is known as the thermometric substance.
|
Devices
|
Property
|
Substance
|
| Liquid-in-Glass Thermometers | Volume (Height) | Mercury, Alcohol |
| Gas Thermometers | Pressure | Helium, Hydrogen |
| Thermocouples | Electromotive Force (emf) |
Copper-Constantan, |
| RTD-Resistance Temperature Devices | Electric Resistance | Platinum, Nickel Copper |
| Thermistor | Electric Resistance | Semiconductors |
|
The gas thermometers are used as a standard worldwide by bureaus of standards and research laboratories.
|
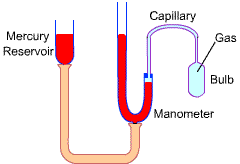 |
|
3.2 Zeroth Law of Thermodynamics
Two systems in thermal equilibrium with a third system are in thermal
equilibrium with each other.
It may seem silly that such an obvious fact is called one of the basic
law of thermodynamics. However, it cannot be concluded from the other
law of thermodynamics, and it serves as a basis for the validity of temperature
measurement.
By replacing the third body with a thermometer, the zeroth law can be
restated as: Two bodies are in thermal equilibrium if both have the same
temperature reading (even if they are not in contact).
Zeroth Law of Thermodynamics
was first formulated by R. H. Fowler in 1931. Since the so-called First
and Second Law of Thermodynamics were formulated much earlier, and since
in a logical development of the subject it must be stated before them,
it has been agreed to designate it as the Zeroth Law.
Temperature scales enable scientists to use a common basis for temperature
measurement. Temperature scales are defined by the numerical values assigned
to standard fixed points.
Examples:
Ice point: a mixture of ice and water which is in equilibrium with
air saturated with vapor at 1-atm pressure.
Steam point: a mixture of liquid water and water vapor, with no
air, in equilibrium at 1-atm pressure.
Triple point: the state of equilibrium among steam, ice and liquid
water.
|
Celsius
Scale
(Centigrade Scale) |
Fahrenheit
Scale
|
||
|
Named
After Swedish Astronomer A. Celsius
|
Named
After German Instrument Maker G. Fahrenheit
|
||
|
Ice
Point
|
0
|
Ice
Point
|
32
|
|
Steam
Point
|
100
|
Steam
Point
|
212
|
|
Triple
Point
|
0.01
|
Triple
Point
|
32.02
|
|
Kelvin
Scale
|
Rankine
Scale
|
||
|
Named
After Lord Kelvin
|
Named
After W. Rankine
|
||
|
Ice
Point
|
273.15K
|
Ice
Point
|
491.67R
|
|
Steam
Point
|
373.15K
|
Steam
Point
|
671.67R
|
|
Triple
Point
|
273.16K
|
Triple
Point
|
491.69R
|
There are no negative temperature on an absolute temperature scale and the lowest attainable temperature is absolute zero.
![]()
At the triple point of water,
T=273.16K (0.01![]() ),
),
![]()
| or | 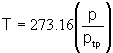 |
[1] |
|
Repeat the measurements
several times with less gas in the bulb in each successive attempt.
For each trial the ratio As pressure decreases,
the |
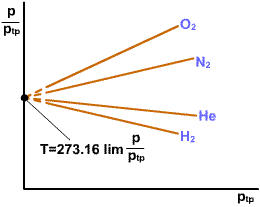 |
|
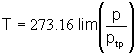
The temperature scale thus defined is independent of the properties of any one gas.
|
R = 1.8 K
Note that the magnitudes
of each division in K and T (K) =
T ( Similarly, T (R) = T
( |
||
If the relation involves temperature differences, it makes no difference and either one can be used. But if the relation involves temperature only instead of temperature difference, then K (the absolute temperature) must be used. When in doubt, it is always safe to use K because there are virtually no situations in which the use of K is incorrect, but there are many thermodynamic relations which will yield erroneous result if