The form of energy that is transferred between two systems (or a system
and its surroundings) by virtue of a temperature difference
| The direction of energy transfer by heat is always from the higher temperature body to the lower temperature one. Once the temperature equality is established, the energy transfer stops. | ||
Sign Convention:
|
Q > 0,
if heat is transferred to a system
Q < 0,
if heat is transferred from a system
Q = 0,
no heat transfer (adiabatic process)
|
||
The sign convention for heat transfer is just the reverse of the one adopted for work.
![]()
Like work, heat is not a property.
The notion of heat at a state has no meaning.
Rate of heat transfer Q
Heat flux ![]()
Three basic modes of heat transfer modes:
(a) Conduction
(b) Convection
(c) Radiation
Energy exchange takes place (from the region of high temperature to that
of low temperature) by the kinetic motion or direct impact of molecules.
|
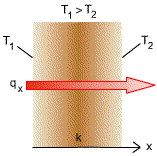
Fourier's Law
where
|
 |
|
|
This law states that the rate of heat flow by conduction is proportional to the area normal to the direction of heat flow and to the temperature gradient in that direction. The minus sign is inserted so that the second law of thermodynamics will be satisfied, i.e., heat must flow downhill on the temperature scale. |
 Example of Conduction |
|
In general, the thermal conductivity is strongly temperature-dependent.
|
Gases
|
-
Collision. - It can be predicted by the kinetic theory of gases. - The thermal conductivity of a gas varies with the square root of the absolute temperature. |
|
|
Liquids
|
-
Collisions. - Same as gases, but more complicated. (since molecules are more closely spaced and molecular force fields exert a strong influence on the energy exchange in the collision process.) |
|
|
Solids
|
-
Lattice vibration and transport by free electrons. - Good electrical conductors are almost always good heat conductors. |
|
Energy exchange takes place as a consequence of the relative motion of
fluid.
Forced Convection - If the fluid motion is artificially induced.
Free (Natural) Convection - If the fluid motion is set up by buoyancy effects resulting from density difference caused by temperature difference in the fluid.
|
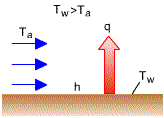
Newton's Law of Cooling
where h: heat transfer coefficient,
|
 |
|
As a result of viscosity, the
velocity of flow will reduce to zero at the wall, the heat must be transferred
only by conduction at that point.
| Heat
Transfer Coefficient For most cases, it is determined by experiments. It varies with: (a) velocity of the flow, (b) physical properties of the fluid, (c) geometry of the body, and (d) position along the surface of the body. |
 Example of Convection |
|
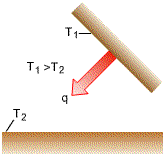
Energy exchange takes place by emission and absorption of electromagnetic
waves.
|
Stefan-Boltzmann Law of Thermal Radiation
This equation is valid only for thermal radiation and it applies only to blackbodies. |
 Example of Radiation |
|
|
|
||
|
2. Radiation Exchange
|
 |
|
1. Heat and Work are boundary phenomena. Both are recognized at the boundaries
of the system as they cross them.
2. Heat and Work are transient phenomena. Systems possess energy, but not heat or work.
3. Heat and Work are not properties. Both are associated with a process, not a state.
4. Heat and Work are functions of path. Their magnitude depends on the path followed during a process as well as the end states.
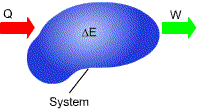
Energy can be neither created or destroyed; it can only change forms.
During an interaction between a system and its surroundings the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
Closed Systems

|
In differential form, In rate form, where E: total energy includes kinetic energy, gravitational potential energy and other forms of energy |
 |
|
All other forms of energy are lumped together as the internal energy.
Internal energy, represented by the symbol U, is an extensive property of the system.
Specific internal energy u = U/m
![]()
![]()
Sensible Energy
The portion of the internal energy that is associated with the kinetic
energies of the molecules.
At the molecular level, the
kinetic energy of a molecule includes
(a) Translational kinetic energy
(b) Rotational kinetic energy
(c) Vibrational kinetic energy
Latent Energy
The portion of the internal energy that is associated with the phase of
a system.
Intermolecular forces are the forces that bind the molecules to each other.
They are strongest in solids and weakest in gases. If sufficient energy
is added to the molecules of a solid or liquid, they will overcome these
intermolecular forces and break away, turning the system to a gas.
Chemical (Bond) Energy
The portion of the internal energy that is associated with the atomic
bonds of a molecule. During a chemical reaction, such as combustion, some
chemical bonds are destroyed while others are formed.
Nuclear Energy
The portion of the internal energy that is associated with the bonds within
the nucleus of an atom.