Admicellar Polymerization
Surfactant
micelles are known to anyone who has washed dishes or themselves (which I
hope is everyone!). Almost all food or dirt is composed of organic matter,
in other words it is oil-like. Therefore, just like oil and water don’t mix
very well, water without soap (the active ingredient in soap is surfactant)
will not wash your dishes or yourself very well, no matter how hard you try
to convince your mother otherwise. So you add soap to water, and by doing
this you can clean whatever it is you wish to clean.
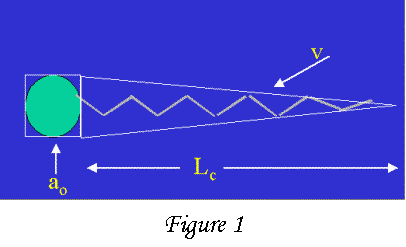
How
does this work, i.e. how does surfactant clean. Surfactants are molecules
that have an oil-like part and a water like part on the same molecule, as
shown in the Figure to the right. The green circle is the water-like part,
while the yellow jagged line is the oil-like part. A typical water-like part
is SO4-Na+ while a typical oil-like part
is composed of 10-20 repeating CH2 units. When you put these molecules
in water, you get many different organizations of these molecules in the water
depending on the packing factor (F) which is defined as F=v/aoLc.The possible
organizations are shown
in the Table below.
| Packing factor |
Arrangement |
| Less than 0.33 |
Spherical, Ellipsoidal
Micelles |
| 0.33-0.5 |
Rod-Like Micelles |
| 0.5-1.0 |
Curved Bilayers |
| 1.0 |
Planar Bilayers |
| Greater than
1.0 |
Reverse Micelles |

In terms of soap, micelles are the important morphology; what happens is that the dirt or food segregates to the inside of the micelles, and is carried away by the running water see Figure 2. If you replace the dirt or food as shown in Figure 2 with monomer, and then polymerize the monomer, you will end up with an emulsion polymer, which is more commonly known as latex paint. In other words, when you paint your walls you are using the same underlying process that you use when you wash your dishes (that's odd, isn't it!!!).
What we can do is to use these adsorbed aggregates as reaction templates, i.e. adsorb a monomer to the interior of the adsorbed aggregates, and allow the monomer to react. This process was discovered approximately 15 years ago at OU and is termed admicellar polymerization. Research concerning surfactant adsorption, admicelles, adsolubililization and reactions within admicelles has progressed steadily in the last 15 years. The work has expanded to include a wide variety of substrates, surfactants, adsolubilizates, reactions and reagents. The fundamentals and possible applications of admicellar polymerization have been and continue to be examined. A few of these fundamentals and applications are discussed in these webpages; consult the references listed below for more information.
The projects on conductive
composites and structural
composites both utilize admicellar polymerization. Using this technology,
we are able to put a very thin layer of polymer on the surface of either a
particulate or fibrous solid, and change the behavior of the material at the
polymer-solid interface. In the project on conductive composites, we are able
to improve electron transfer across the particle-particle interface which
increases the conductivity of the composite. In structural composites we are
able to improve polymer-filler adhesion and hence get better mechanical properties.
We have also recently begun more fundamental work. We have addressed the question:
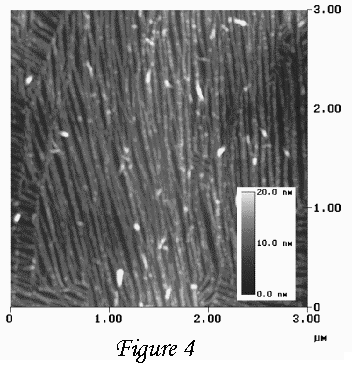
Is it possible to transfer the morphologies shown in Figure 3 to the polymer.
Specifically, we have used the cylindrical and hemi-cylindrical morphologies
to produce polymer wires as shown in Figure 4. We call these structures wires
because we have formed an electrically conducting polymer, polyaniline, to
form these structures. Hence, we believe this technique might be a way to
pattern wires on the surface of atomically flat solid, i.e. single crystal
silicon as is used in semiconductor manufacture. We have not yet published
this result, but the pictures sure are pretty neat.
See the webpage on nanostructures to see how we use admicellar polymerization along with templated surfaces to create unique polymer nanostructures.
Relevant References
W.B. Genetti, W.L. Yuan, B.P. Grady, E.A. O'Rear, C.L. Lai and D.T. Glatzhofer, "Polymer Matrix Composites: Conductivity Enhancement through Polypyrrole Coating of Nickel Flake", Journal of Material Science, 33, 3085 (1998).
B.P. Grady, E.A. O'Rear, L. Penn and A. Pedicini, "Admicellar Polymerization of Styrene-Isoprene on Glass Cloth for Use in Composite Manufacture" Polymer Composites, 19, 579 (1998).
H.J. Barraza, M.J. Hwa, K. Blakely, E.A. O'Rear, and B.P. Grady, "Wetting Behavior of Elastomer-Modified Glass Fibers", Langmuir, 17, 5288 (2001).
J.H. O'Haver, B.P. Grady, E.A. O'Rear and J.H. Harwell, "Admicellar Polymerization", in Reactions and Synthesis in Surfactant Systems (Surfactant Science, Vol. 100), J. Texter ed. (New York, Marcel Dekker) 2001.
W. Yuan, E.A. O'Rear, B.P. Grady and D.T. Glatzhofer, "Nanometer-Thick Polypyrrole Films Formed by Admicellar Polymerization under Conditions of Depleting Adsolubilization", Langmuir, 18, 3343 (2002).
K. Bunsomit, R. Magaraphan, E.A. O'Rear and B. P. Grady, "Polypyrrole-Coated Natural Rubber Latex by Admicellar Polymerization", Colloid and Polymer Science, 280, 509 (2002).
X. Wei, A.D.W. Carswell, W. Alvarez and B.P. Grady, “X-ray Photoelectron Spectroscopic Studies of Hydrophilic Surfaces Modified via Admicellar Polymerization, Journal of Colloid and Interface Science, 264, 296 (2003).
A.D.W. Carswell, E.A. O’Rear, B.P. Grady, “Adsorbed Surfactants as Templates for the Synthesis of Morphologically Controlled Polyaniline and Polypyrrole Nanostructures on Flat Surfaces: From Spheres to Wires to Flat Films”, Journal of the American Chemical Society, 125, 14793 (2003).
K. Blakely, B.P. Grady, H.J. Barrazza and E.A. O'Rear, “Effects of Styrene-Isoprene Copolymer Glass Fiber Coatings on Woven Glass Cloth Epoxy Composite Performance”, Polymer Composites, 25, 82 (2004).
P. Lekpittaya, N. Yanumet, B.P.Grady, and E.A. O’Rear, “Resistivity of Conductive Polymer-Coated Fabric”, Journal of Applied Polymer Science, 92, 2629 (2004).
P. Rungruang, B.P. Grady and P. Supaphol, “Surface-Modified Calcium Carbonate Particles by Admicellar Polymerization to be used as Filler for Isotactic Polypropylene, Colloids and Surfaces A: Physicochem. Eng. Aspects, 275, 114 (2006).