Commercial introduction of a polymer made from a new monomer is an extremely expensive, and risky, enterprise. One strategy to introduce new products into the market without a large investment is to take two or more different polymers and blend them together to make a new product with unique properties. However, the success of this strategy has been limited because the mechanical properties of the blend are typically far worse than a simple mixing law would predict. This situation arises because most polymers are not miscible in one another. Hence, the typical result of mixing two polymers together is a material that consists of two separate phases, and the interface between the phases is very weak since polymer chains do not crossover the boundaries because of the aforementioned incompatibility.
Probably the most effective strategy to reduce this problem of immiscibility is to design blends so that a reaction can occur at the interface. The operative term in this last sentence is reduce; in other words certain mechanical properties, in particular toughness, are still below what would be predicted by a mixing rule. Our focus is on blend systems that have such reactions that occur at the interface. In particular, polyamides inherently have many attractive properties, and can react with many functional groups because of the terminal primary amines, and, to a lesser extent, the possibility of chemical interchange reactions involving the amide linkage. Hence, polyamides have been extensively studied as blend components.
Our work combines our expertise with ionomers and extends it to blend systems with polyamides. The carboxylic acid is ideal for reacting with the primary amine, and much research throughout the years has gone into investigating ethylene-acid copolymers as blends with polyamides, and as blend compatibilizers for nylon/polyethylene blends. Although circumstantial evidence suggested this was the case, we have shown that zinc-neutralized materials form better blends than sodium neutralized materials, as shown by the smaller dispersed phase sizes as shown in the table below.
|
Nylon/Ionomer Ratio |
Dispersed Phase Size sodium ionomer (microns) |
Dispersed Phase Size Zinc Ionomer (microns) |
20/80 |
1.6 |
0.8 |
40/60 |
2.2 |
0.5 |
50/50 |
1.9 |
0.6 |
60/40 |
1.9 |
0.4 |
80/20 |
0.8 |
0.2 |
However, in another paper, we compared nylon/LDPE blends compatibilized with ionomers neutralized with sodium, zinc or lithium or the acid copolymer. In all cases, the ionomers did a much better job at compatibilization than the acid copolymer, but there was no significant difference between the different cations.
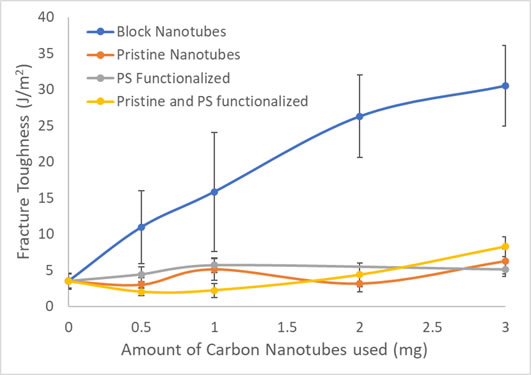
A third aspect of our work combines our expertise in polymer blends with our expertise in nanotubes. We have made diblock nanotubes with two different chemistries and used these to compatibilize a model system: namely polystyrene-poly(methyl methacrylate) blends. The graph below shows the results compared to model systems including using mixtures of functionalized and unfunctionalized tubes at the same ratio that occurs in the block tubes.

.
P. Leewajanakul, R. Pattanaolarn, J. W. Ellis, M. Nithitanakul and B.P. Grady, "The Use of Zinc-Neutralized Ethylene-Methacrylic Acid Copolymer Ionomers as Blend Compatibilizers for Nylon 6 and Low Density Polyethylene", Journal of Applied Polymer Science, 89, 620 (2003).
N. Pongrakananon, N. Somrang, W. Visitsart, P. Supaphol, M. Nithitanakul and B. P. Grady, “Blends of Ethylene-Methyl Acrylate-Acrylic Acid Terpolymers with Ethylene-Acrylic Acid Copolymers: Mechanical and Thermomechanical Properties”, Journal of Applied Polymer Science, 91, 2216 (2004).
A. Lahor, M. Nithitanakul, B.P. Grady, “Blends of Low-Density Polyethylene with Nylon Compatibilized with a Sodium Neutralized Carboxylate Ionomer” European Polymer Journal, 40, 2409 (2004).
B. Chatreenuwat, M. Nithitanakul, and B.P. Grady, “The Effect of Zinc Oxide Addition on the Compatibilization Efficiency of Maleic Anhydride Grafted High-Density Polyethylene Compatibilizer for High-Density Polyethylene/Polyamide 6 Blends”, Journal of Applied Polymer Science, 103, 3871 (2007).
W. Sinthavathavorn, M. Nithitanakul, R. Magaraphan, B.P. Grady, “Blends of Polyamide 6 with Low-Density Polyethylene Compatibilized with Ethylene-Methacrylic Acid Based Copolymer Ionomers: Effect of Neutralizing Cation”, Journal of Applied Polymer Science, 107, 3090 (2008).
W. Sinthavathavorn, M. Nithitanakul, B.P. Grady, R. Magaraphan, “Melt rheology of low-density polyethylene/polyamide 6 using ionomer as a compatibilizer”, Polymer Bulletin, 61, 331 (2008)
W. Sinthavathavorn, M. Nithitanakul, B.P. Grady, R. Magaraphan, “Melt Rheology and Die Swell of PA6/LDPE blends by using lithium ionomer as a compatibilizer”, Polymer Bulletin, 63, 23 (2009).
S. Charoenpongpool, M. Nithitanankul and B.P. Grady, “Melt-neutralization of maleic anhydride grafted on high-density polyethylene compatibilizer for polyamide-6/high-density polyethylene blend: effect of neutralization level on compatibility of the blend” Polymer Bulletin, 70, 293 (2013).
J. Guo, N. Briggs, S. Crossley, B.P. Grady, “Morphology of polystyrene/poly(methyl methacrylate) blends: Effects of carbon nanotubes aspect ratio and surface modification”, AICHE Journal, 61, 3500 (2015)
J. Guo, N. Briggs, S. Crossley, B.P. Grady “A new finding for carbon nanotubes in polymer blends: Reduction of nanotube breakage during melt mixing”, Journal of Thermoplastic Composite Materials, 31, 110 (2018).
L. Barrett, F. Ide Seyni, M.R. Komarneni, J.A. Zapata-Hincapie, D.T. Glatzhofer, B.P. Grady and S. Crossley, “Anisotropically Functionalized Nanotube Anchors for Improving the Mechanical Strength of Immiscible Polymer Composites”, ACS Applied Nano Materials, 4, 580 (2021).
F. Ide Seyni, L. Barrett, S. Crossley and B.P. Grady, “Polystyrene and poly(methyl methacrylate) interfaces reinforced with diblock carbon nanotubes”, Polymer Engineering and Science, 61, 1186 (2021).