Diagram of E-MAA
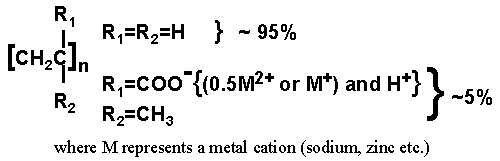
Ionomers belong to the class of polymers known as specialty polymers. Specialty polymers are used whenever the high cost of the raw material relative to commodity polymers can be compensated by the unique properties of the resulting product. Typically, specialty polymers are used when the product must be resistant to some external stimuli, i.e. mechanical, thermal or chemical. The market for these polymers has exploded in the last 20 years with the biggest users being the auto, aerospace and sporting goods industries.
Ionomers are a family of materials where a small number, typically less than 10 mole percent, of the polymer repeat units have an ionic functionality covalently bonded to the polymer backbone. The introduction of a small amount of ionic groups improves the material properties of the polymer dramatically, resulting in an increased modulus, improved abrasion and tear resistance and increased impact strength. In addition, as ionomers are not thermosetting materials, they can be processed as thermoplastics using conventional equipment. This combination of excellent properties and processing ease has led to the use of ionomers in many high performance material applications including golf ball covers, food packaging and roofing materials.
Surlyn from the DuPont Corporation is one of the most important commerical ionomers. DuPont is one of the sponsors of our research, and Surlyn has been investigated. Surlyn is a copolymer of ethylene and methacrylic acid.
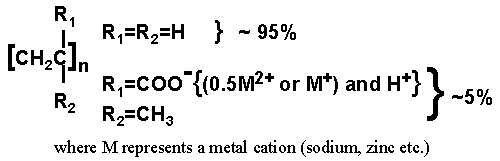
Other important commercial ionomers include Nafion, which is sulfonated tetrafluorethylene ionomer and is used as a membrane separator, and sulfonated polystyrene, which is used as a viscosity modifier.
Ionomers have significantly better properties than the un-ionized precursor because the ionic groups phase separate into ion-rich domains. The domains are thought to be spherical with a diameter on the order of 1 nanometers and an interdomain spacing on the order of 5 nanometers. The focus of our research is to understand what the structure is of these domains. A complete answer requires one to determine the intradomain atomic arrangement, as well as to determine the interdomain arrangement. To determine the former, we use extended x-ray absorption fine structure (EXAFS) to determine the latter, we use small-angle x-ray scattering. To determine more about our work in this area, please consult the papers listed below.
B.P. Grady, J.A. Floyd, W.B. Genetti, P. Vanhoorne and R.A. Register, "X-ray Absorption Spectroscopy Studies of Zinc Neutralized Ethylene-Methacrylic Ionomers" Polymer, 40, 283 (1999).
B.P. Grady, "Effect of Co-Neutralization on Internal Aggregrate Structure in Ethylene-Based Ionomers" Macromolecules, 32, 2983 (1999).
A. Welty, S. Ooi, and B.P. Grady, "Effect of Water on Internal Aggregate Structure in Zinc-Neutralized Ionomers" Macromolecules, 32, 2989 (1999).
B.P. Grady, "Relative Size of Ionic Aggregates Determined by X-ray Absorption Spectroscopy" Polymer, 41, 2325-2328 (2000).
K.V. Farrell and B.P. Grady, "Studies of Cation Local Environment in Sodium-Neutralized Ethylene Copolymer Ionomers", Macromolecules, 34, 7108 (2001).
P. Leewajanakul, R. Pattanaolarn, J. W. Ellis, M. Nithitanakul and B.P. Grady, "The Use of Zinc-Neutralized Ethylene-Methacrylic Acid Copolymer Ionomers as Blend Compatibilizers for Nylon 6 and Low Density Polyethylene", Journal of Applied Polymer Science, 89, 620 (2003).
N. Pongrakananon, N. Somrang, W. Visitsart, P. Supaphol, M. Nithitanakul and B. P. Grady, “Blends of Ethylene-Methyl Acrylate-Acrylic Acid Terpolymers with Ethylene-Acrylic Acid Copolymers: Mechanical and Thermomechanical Properties”, Journal of Applied Polymer Science, 91, 2216 (2004).
N. Somrang, M. Nithitanakul, P. Supaphol and B.P. Grady, “Non-Isothermal Melt Crystallization Kinetics for Ethylene-Acrylic Acid Copolymers and Ethylene-Methyl Acrylate-Acrylic Acid Terpolymers”, European Polymer Journal, 40, 829 (2004).
B.P. Grady, J.G.P. Goossens and M.E. Wouters “The Morphology of Zinc-Neutralized Maleated Ethylene-Propylene Copolymer Ionomers: The Structure of Ionic Aggregates as Studied by X-ray Absorption Spectroscopy” Macromolecules, 37, 8585 (2004).
W. Sinthavathavorn, M. Nithitanakul, R. Magaraphan, B.P. Grady, “Blends of Polyamide 6 with Low-Density Polyethylene Compatibilized with Ethylene-Methacrylic Acid Based Copolymer Ionomers: Effect of Neutralizing Cation”, Journal of Applied Polymer Science, 107, 3090 (2008).
B.P. Grady, “Review and Critical Analysis of the Morphology of Random Ionomers Across Many Length Scales” Polymer Engineering and Science, 48, 1029 (2008
W. Sinthavathavorn, M. Nithitanakul, B.P. Grady, R. Magaraphan, “Melt Rheology of Low-density polyethylene/polyamide 6 using Ionomer as a Compatibilizer”, Polymer Bulletin, 61, 331 (2008).
W. Sinthavathavorn, M. Nithitanakul, B.P. Grady, R. Magaraphan, “Melt Rheology and Die Swell of PA6/LDPE blends by using lithium ionomer as a compatibilizer”, Polymer Bulletin, 63, 23 (2009).
Y. Rui, B.P. Grady, "Long-time crystallization kinetics in zinc-neutralized ethylene-methacrylic acid ionomers", Thermochimica Acta, 565, 183 (2013).
N. El Choufi, S. Mustapha, A.R. Terhani-Bagha, B.P. Grady, "Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization" Polymers, 14, Art. 3575 (2022).
B.P. Grady, "Very long-time crystallization kinetics for ethylene-methacrylic acid ionomers neutralized with zinc, sodium, lithium and magnesium", Thermochimica Acta, 734, Art. 179694 (2024).